日前,褀氏生物自主研发的新冠抗体检测试剂,进入了中国医药保健品进出口商会的“白名单”,同时也获得多个国家的准入资质,其中血清总抗体检测法作为WHO推荐的方法学,适合大规模人群快速自动化检测。褀氏为继续支持国际社会抗击疫情,贡献一份中国力量!
The two types of novel coronavirus antibody test reagents independently developed by Kisshealth's have entered the "White List" of China Chamberof Commerce for Import & Export of Medicines & Health Products. At the same time, we have also won access qualifications from a number of countries. As a methodology recommended by WHO, Serum total antibody method is suitable for rapid and automatic detection of large-scale population. Kisshealth contributes to China's efforts to continue to support the international community in the fight against the epidemic.
在全球疫情持续蔓延的特殊时期,为更有效支持国际社会共同应对全球公共卫生危机,现就进一步加强防疫物资质量监管、规范出口秩序。2020年4月25日,国家商务部、海关总署、国家市场监督管理总局发布公告2020年第12号《关于进一步加强防疫物资出口质量监管的公告》。
In order to more effectively support the international community's joint response to the global public health crisis at this particular time when the global epidemic continues to spread, it isimportant to further strengthen the role of the international community inaddressing the global public health crisis. Strengthening quality control of epidemic prevention materials and regulating export order. On April 25, 2020,the Ministry of Commerce, the General Administration of Customs, the National The General Administration of Market Supervision and Administration issued Announcement No. 12 of 2020 “Announcement on Further Strengthening the Quality Control of Export of Epidemic Prevention Materials” .
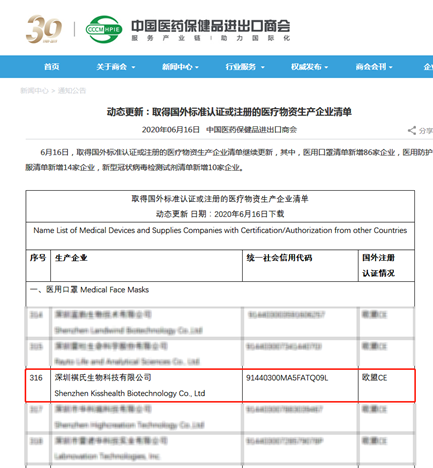
褀氏生物入选中国医药保健品进出口商会,新冠试剂出口“白名单”
新冠疫情爆发以来,褀氏生物迅速组织公司研发力量,针对新型冠状病毒启动攻关,经过研发团队不懈努力,克服一切困难,顺利完成了新型冠状病毒总抗检测试剂的开发。
Since the outbreak of the novel coronavirus epidemic, Kisshealth has rapidly organized its research and development team to tackle the novel coronavirus and overcome all the difficulties to successfully complete the development of the novel coronavirus total antibody test.
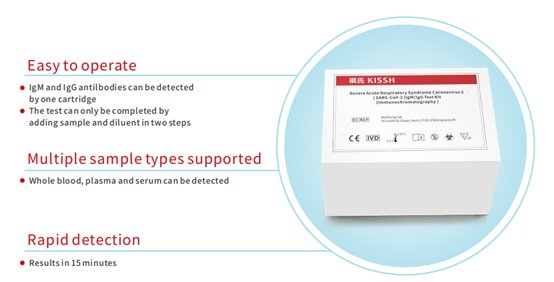
褀氏的新冠病毒检测试剂盒既适合单独诊所进行测试,也适合大型医院。适合独立诊所使用的是胶体金法,适合大规模人群筛查的是免疫比浊法。
Kisshealth's novel coronavirus test kit are suitable for testing not only in individual clinics, but also in large hospitals. Colloidal gold method issuitable for individual clinic, and immune turbidimetric method is suitable forlarge-scale population screening.

免疫比浊法检测试剂盒是自动化方法学,可配套医院现有自动化设备,可应用于世界各大品牌的生化仪器,如贝克曼、罗氏、雅培等等,降低了医护人员检测过程中被感染的风险,可以实现上千人份同时测试,适于大型三甲医院至基层医疗机构进行新型冠状病毒的大规模筛查检测。测试速度可达2000测试/小时,重复性<3%,批间差 <5%,适用于疑似患者隔离区、确诊患者隔离区、三甲医院发热门诊、基层医院、社康门诊等多种不同的复杂应用场景。
The immunoturbidimetric test kit is an automatic methodology, whichcan be equipped with existing automatic equipment in hospitals, and can beapplied to biochemical instruments of major brands in the world, such as Beckman, Roche, Abbott, etc., reducing the risk of healthcare workers beinginfected during testing, and can achieve thousands of simultaneous testing,which is suitable for large-scale screening and testing of novel coronavirusfrom large-scale third-class hospitals to primary care institutions. The testspeed can reach 2000 tests per hour, reproducibility <3%, inter-batchvariation <5%. It is suitable for a variety of complex applicationscenarios, such as isolation area for diagnosed patients, fever clinics intertiary hospitals, primary hospitals, and community health clinics.
新型冠状病毒检测试剂盒(胶乳增强免疫比浊法)的优势:
1、拥有自主研发胶乳抗原偶联技术、液相胶乳增强免疫比浊法体外诊断技术;
2、样本为血液,来源灵活,降低医护人员的暴露感染风险,极大缓解临床诊疗压力;
3、采用目前国内最快的液相方法学,突破对人员场所的限制,缩短检测用时,操作便捷;
4、适用面广泛,可应用医疗机构广泛,与医院全自动生化分析仪配套使用,对于广大基层医疗机构来讲经济负担轻、成本可控、兼容性强;
5、可与核酸检测、CT检查配套进行精准诊断,实现疑似患者的快速诊断和大规模密切接触人群的快速筛查,防止疫情扩散。
生化检测及其他指征进行综合判读、联合应用,更能有助于提高疾病的检出率,尽可能地找出确诊患者,更有利于疫情的控制。
Advantagesof novel coronavirus test kit (latex enhanced immune turbidimetry):
1. It has self-developedlatex antigen coupling technology and liquid phase latex enhancedimmunoturbidimetry In-vitro diagnosis technology;
2. The sample is blood, thesource is flexible, reduce the risk of exposure and infection of medical staff,greatly relieve the pressure of clinical diagnosis and treatment;
3. It adopts the fastestliquid phase method, breaks through the restrictions on personnel places,shortens the detection time and is easy to operate ;
4. It can be widely used inmedical institutions, and it is used in combination with the hospital automaticbiochemical analyzer. For the majority of primary medical institutions, it hasthe advantages of light economic burden, controllable cost and strongcompatibility;
5. It can be used forprecise diagnosis in combination with nucleic acid detection and CT examinationto realize rapid diagnosis of suspected patients and rapid screening oflarge-scale close contact population, so as to prevent the spread of theepidemic.
近期,国内连续几天出现本土新增确诊病例,一方面提醒着我们要倍加珍惜来之不易的防控成果,另一方面也为我们敲响了警钟。根据世界实时统计数据显示,截至北京时间6月17日15时27分,全球新冠肺炎累计确诊病例超过827万例,其中海外达到8181606例,累计死亡病例超过44.15万例,达到429758例。
Recently,there have been new confirmed cases in China for several consecutive days, which on the one hand reminds us to cherish the hard-won results of preventionand control, on the other hand, it also rings the alarm for us. According to real-time world statistics, as of 15:27 on June 17, Beijing time, thecumulative number of confirmed cases of COVID-19 worldwide exceeded 8.27million, of which 8181606 were overseas, and the cumulative number of deaths exceeded 441500, reaching 429758.
面对国际疫情,褀氏生物承担起企业应有的责任和社会担当,谨记责任勇于担当,时刻为疫情抗争贡献自身力量,褀氏所研发的新冠检测试剂将为全球抗疫战构筑抵御病毒的卫生防线。褀氏用行动践行着构建人类命运共同体的庄严承诺。
In the face of the international epidemic, Kisshealth assumes its due responsibility and social responsibility, bear in mind that it has the courage to take responsibility, always contribute its own strength to the fight against the epidemic. Kisshealth will build a health line of defense against the virus for the global anti-epidemic war. With actions, Kisshealth fulfills the solemn promise of building a community with a shared future for mankind.


 地址:深圳市龙华区景龙建设路金苹果304
地址:深圳市龙华区景龙建设路金苹果304